Researchers are exploring how PROteolysis TArgeting Chimeras (PROTACs) can potentially treat cancer, neurodegenerative diseases, and other difficult-to-treat conditions. PROTACs are heterobifunctional small molecules that help engage the ubiquitin-proteosome system for protein degradation and are considered a subset of targeted protein degraders (TDPs). They consist of a ligand-linker-recruitment moiety and unite the protein that needs to be degraded with the E3 ligase. This eventually leads to the degradation of that protein with the PROTAC being recycled to repeat the process with another protein molecule.
Variations of PROTACs include:
- PROteolysis Targeting AntiBodies (PROTABs)
- Molecular glues
- Lysosome-targeting chimeras (LYTACs)
- Macrophage degradation targeting chimeras (MADTACs)
We analyzed the CAS Content CollectionTM, the largest human-curated collection of scientific information, to better understand how the research landscape has changed in recent years, and we found a remarkable increase in both journal and patent publications relating to PROTACs in the last few years (see Figure 1). Our analysis also revealed which PROTACs are gaining traction with researchers, and the diseases they are being used to target most.

How PROTACs target proteins
Protein degradation is a normal physiological process to eliminate damaged or misfolded proteins. PROTACs facilitate this process by acting as a bridge between protein targets of interest (i.e., those playing a role in cancer and other diseases) and E3 ligases. These ligases tag the protein of interest with ubiquitin; the ubiquitin tags act as a signal to the ubiquitin-proteosome system that the protein molecule is to be degraded. PROTACs bring these ligases into close proximity with protein targets, enabling ubiquitination and subsequent degradation (see Figure 2).

“PROTAC: Proteolytic Targeting Chimera”. Retrieved from https://app.biorender.com/biorender-templates.
The PROTAC molecule itself is composed of a ligand-linker-recruitment moiety with the ligand acting as a means to target the protein of interest, the linker allowing for optimal spacing between the protein and E3 ligases, and the recruitment moiety allowing the engagement of appropriate E3 ligases.
Initially, PROTACs were composed of a ligand-linker-recruitment moiety and targeted one protein of interest. However, new developments have resulted in different PROTAC designs that can target proteins in numerous ways:
- Dual and multi-target PROTACs: Unlike conventional PROTACs, these can be designed to target more than one type of protein.
- Peptide-based PROTACs: These utilize peptide-based moieties in place of small molecules as the targeting ligand in conventional PROTACs. This allows them to target proteins that do not have existing small molecule inhibitors.
- bioPROTACs: These consist of engineered E3 ligases with an attached protein that functions like the targeting ligand in conventional PROTACs and interacts with the protein of interest.
- In-cell click-formed proteolysis targeting chimeras (CLIPTACs): CLIPTACs have been pursued to try to overcome issues with oral drug administration of PROTACs. They form the PROTAC within cells by administration of the targeting ligand and E3 recruitment moiety one after the other, and the in-cell coupling uses an inverse electron demand Diels–Alder (IEDDA) cycloaddition reaction between tetrazine attached to the E3 recruitment moiety and trans-cyclo-octene attached to the targeting ligand.
- Click-release PROTACs (crPROTACs): These use the IEDDA reaction similar to CLIPTACs. The idea is to administer a prodrug version of PROTAC which releases the active version at only desirable areas/sites.
- PHOtochemically TArgeting Chimeras (PHOTACs): These achieve maximal protein degradation upon light activation, conferring selectivity and potentially reducing off-target effects.
E3 ligases generate research interest
As E3 ligases are recruited by PROTACs, we analyzed the nearly 2,000 PROTAC-related documents in the CAS Content Collection and found four E3 ligases that are of particular interest to researchers (see Figure 3).
- Cereblon: This is a protein composed of about 440 amino acid residues that interacts with numerous proteins to form the E3 ubiquitin ligase complex. Cereblon is considered the most-explored E3 ligase in relation to PROTACs.
- VHL: This acts as the substrate recognition receptor/subunit for CRL2, a cullin-RING E3 ligase (CRL) complex, in conjunction with elongin B, elongin C, cullin 2, and RING box protein 1 (Rbx1).
- IAP: This is a family of proteins including cellular IAP1 (cIAP1), cIAP2, and X-linked IAP (XIAP). PROTACs dependent on IAP recruitment are often referred to as specific and nongenetic IAP-dependent protein erasers (SNIPERs).
- MDM2: This is a protein composed of about 480 amino acid residues whose overexpression has been linked to resistance against chemotherapy.

We identified co-occurrences in the literature between E3 ligases, substrate recognition receptors, and protein targets (see Figure 4). Cereblon and VHL most often co-occur with bromodomain-containing proteins. Other frequent co-occurrences with cereblon include B-cell leukemia and lymphoma proteins and androgen receptors (ARs) and estrogen receptors (ERs). MDM2 and IAP show an even distribution across protein targets:
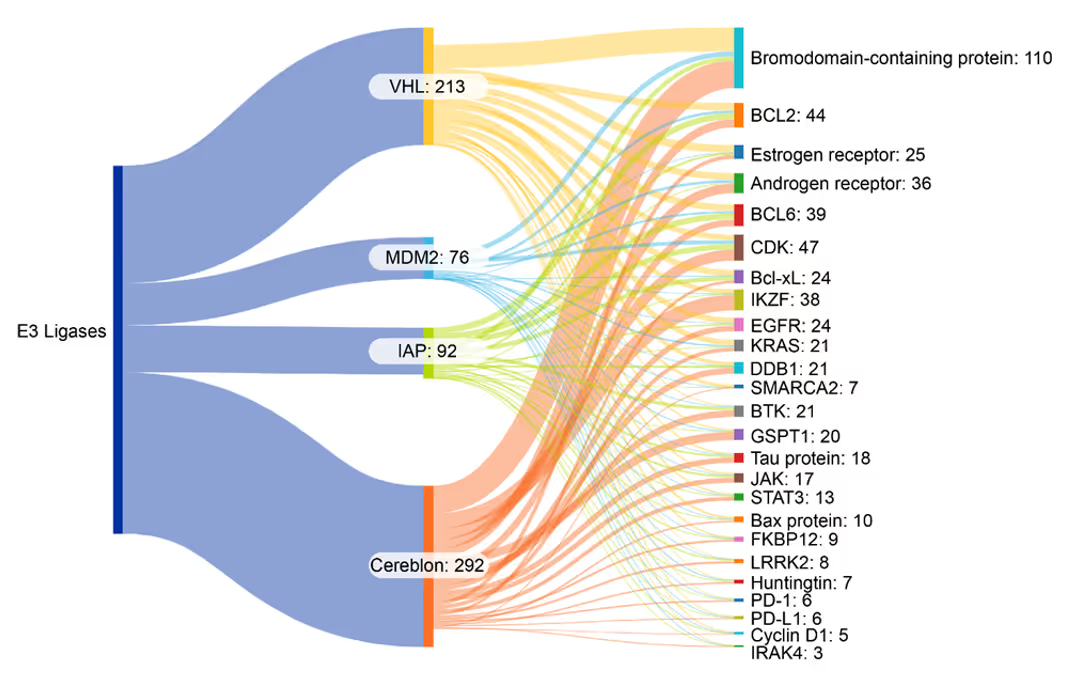
(right column) in PROTAC-related publications. Source: CAS Content Collection for the period 2003-2024.
PROTACs target cancer, infectious diseases, and neurodegenerative conditions
Our analysis shows that cancer remains the leading disease targeted by PROTACs research, followed by infectious diseases and neurodegenerative conditions (see Figure 5).
Breast cancer, prostate cancer, and melanoma are the most common solid cancers co-occurring with PROTACs, and the recent breakthroughs targeting ARs in prostate cancer and ERs in breast cancer explain the prevalence of these diseases in the literature.

CAS Content Collection for the period 2003-2024.
Neurodegenerative diseases like Alzheimer’s and Parkinson’s, which involve misfolded proteins, may also be targeted by protein-degrading PROTACs. If these small molecule drugs can successfully destroy the defective proteins driving these diseases, it could signal important treatment breakthroughs that address the causes, not just the symptoms. Tau protein, for example, has a growing number of publications in the PROTAC field (see Figure 6), demonstrating the increased interest in developing these drugs for neurodegenerative conditions.
Other common protein targets include ARs, ERs, and bromodomain-containing proteins, but also proteins that have resisted treatment with small molecule drugs thus far, such as KRAS and BCL2. These efforts have yet to produce clinical candidates since most are for AR and ER, but they are offering new possibilities in targeting these proteins that play important roles in various cancers.

PROTAC clinical trials are growing rapidly
Since 2020, the number of drugs in the preclinical stage of development has increased more than four-fold. As of 2024, there were six PROTACs in Phase I and Phase II clinical trials each (see Figure 7). This is remarkable considering that the earliest journal publication related to PROTAC can be traced back to 2001 – little more than two decades ago.
Most of these trials are targeting unspecified forms of cancer, but breast and prostate cancer feature prominently as well. Drugs for these latter diseases have reached Phase II and Phase III. For example, vepdegestrant (ARV-471), a PROTAC designed by Arvinas and Pfizer for treating breast cancer, has reached Phase III (ref). GT20029, the first topical PROTAC designed by Suzhou Kintor Pharmaceutical, is another promising treatment targeting AR that has reached Phase II.
Preclinical trials are also underway for protein targets involved in Alzheimer’s, Parkinson’s, and Huntington’s disease, as are kinases involved in HIV, hepatitis B, and autoimmune diseases. The variety of targets being explored for treatment with PROTACs demonstrates the potential for these drugs to tackle numerous hard-to-treat conditions.


Overcoming challenges to clinical usage of PROTACs
While PROTACs are certainly very promising, their development is still early, and they have important challenges that must be addressed before they can enter widespread clinical usage. One key development will be ensuring PROTACs only bind to target proteins in damaged or unhealthy cells. Off-target effects where PROTACs degrade proteins in healthy cells can be very harmful. A potential solution to this issue is designing novel types of PROTACs such as PHOTACs and CLIPTACs, which are likely to allow the spatiotemporal release of active PROTACs.
Characterization is another challenge — most characterization techniques focus on testing whether the designed PROTACs bind to a protein of interest but do not test or consider interactions between PROTACs and the protein of interest within a live cell. This incomplete knowledge prevents a deeper understanding of the point at which PROTACs might be failing.
Lastly, since PROTACs tend not to comply with Lipinski’s rule of five, designing them to be administered orally is difficult. Developing new formulations and drug delivery systems will be necessary to overcome this obstacle.
These advances will take intense research efforts, but progress with non-traditional modalities like covalent inhibitors is demonstrating that new approaches can make a meaningful difference in new treatments. With so much to gain in targeting the proteins driving cancer and other diseases, the scientific community is wise to invest time and effort to unlock these breakthroughs.







